Atomic Structure (Models of the Atom)
 |
Atomic Structure (Models of the Atom) |
Here you will learn about different atomic models that completely describe the structure of the atom, these are Dalton’s atomic theory, J.J. Thomson, Ruther Ford, Bohr, and the Quantum mechanical model of the atom.
Rutherford Atomic Model
Rutherford Atomic Model - J. J. Thomson's "plum
pudding" model was unable to account for some experimental findings
related to the atomic structure of elements. British scientist Ernest
Rutherford carried out an experiment, and using the results of this experiment,
he proposed Rutherford's Atomic Model and explained the atomic structure of the
elements.
Rutherford's model revolutionized the understanding of atomic structure by introducing the idea of a dense, positively charged nucleus and the existence of empty space within atoms.
In an experiment, Rutherford bombarded a thin sheet of
gold with -alpha particles and then tracked the paths taken by the particles
after they made contact with the foil.
In his experiment, Rutherford fired high-energy beams
of -particles at a 100 nm-thick gold sheet, coming from a radioactive
source. He surrounded the thin gold foil with a fluorescent zinc sulfide screen
in order to analyze the deflection the -particles experienced. Rutherford
stated certain findings that were at odds with Thomson's atomic model.
 |
| Rutherford atomic model |
Postulates:
The Rutherford atomic model states:
The atom consists of a tiny, positively charged nucleus at the center.
The nucleus contains most of the atom's mass, but occupies very little space.
Electrons, which are negatively charged, orbit around the nucleus in circular paths.
Most of the atom is empty space.
An atom's positive charge and the majority of its mass
are packed into a very small volume. Using the term "nucleus," he
described this area of the atom.
According to Rutherford's theory, an atom's nucleus is
surrounded by electrons that are negatively charged. He also asserted that the
electrons that surround the nucleus travel in a circular pattern at extremely
high speeds. He gave these elliptical routes the name orbits.
A powerful electrostatic force of attraction holds the
negatively charged electrons and the positively charged mass of particles that
makes up the nucleus together.
Limitations:
Despite being founded on experimental evidence, the
Rutherford atomic model proved unable to account for some phenomena.
According to Rutherford, the electrons go along
predetermined routes known as orbits as they circle the nucleus. An electron
rotating around the nucleus should create electromagnetic radiation because,
according to Maxwell, accelerating charged particles emit such radiation. This
radiation would transport energy from the electron's speed at the expense of the
orbits getting smaller. In the nucleus, the electrons would eventually
collapse. According to calculations, an electron would enter the nucleus
according to the Rutherford model in less than 10-8 seconds. So the Rutherford
model could not account for the stability of an atom and did not agree with
Maxwell's theory.
One of the problems with the Rutherford model was that
it was an incomplete theory because it said nothing about how the electrons
were arranged in an atom.
The early atomic models, albeit erroneous and unable
to account for some experimental findings, served as the foundation for later
advances in the field of quantum mechanics.
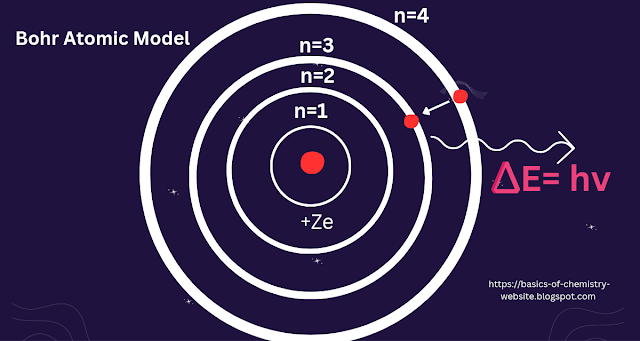
Bohr atomic model:
Neil Bohr put forth the Bohr model of the atom in
1915. It was created by the modification of Rutherford's atomic model. In his
nuclear model of an atom, Rutherford showed how a positively charged nucleus is
surrounded by negatively charged electrons.
Bohr's hypothesis is applicable to entities that are
similar to hydrogen but only have one electron, such Li2+. Atoms similar to
hydrogen are amenable to Bohr's hypothesis (single electron system). Only one
electron makes up Li2+ and the H atom. He and He2+ each have two and zero
electrons.
The Bohr model has undergone numerous revisions, most
notably the Sommerfeld model, often known as the Bohr-Sommerfeld model, which
proposed that electrons orbit a nucleus in elliptical orbits rather than the
circular orbits predicted by the Bohr model. The Bohr-Sommerfeld system was
fundamentally inconsistent, which led to a number of paradoxes.
Bohr's model was significant as it explained the discrete emission or absorption spectra observed in certain experiments, such as the hydrogen line spectrum. It introduced the concept of quantized energy levels and provided a framework for understanding the behavior of electrons within the atom.
Postulates
Electrons move in specific circular orbits around the nucleus, known as energy levels.
Electrons can jump between energy levels by absorbing or emitting energy.
Electrons in stable orbits do not emit radiationIn an atom, negatively charged electrons travel in fixed circular paths around the positively charged nucleus that are referred to as orbits or shells.
These circular orbits are referred to as orbital
shells because each orbit or shell has a defined energy.
The quantum number (n=1, 2, 3, etc.), which is an
integer, is used to indicate the energy levels. The lowest energy level in this
quantum number range, n=1, lies on the nucleus side. When an electron reaches
the lowest energy level, it is referred to as being in the ground state. The
orbits n=1, 2, 3, 4... are allocated as K, L, M, N.... shells.
The energy needed to transfer an electron in an atom
from one energy level to another is acquired, and the energy needed to move an
electron from one energy level to another is lost.
Limitations
Zeeman Effect could not be explained by Bohr's atomic
model (effect of magnetic field on the spectra of atoms).
The Stark effect was also not adequately explained
(effect of electric field on the spectra of atoms).
The Heisenberg Uncertainty Principle is broken.
The spectra obtained from bigger atoms could not be explained by it.
Quantum mechanical model
The quantum mechanical model, developed in the 1920s, is the most advanced and widely accepted model of the atom. It is based on quantum theory.
Schrödinger's wave equation and its solution serve as
the foundation for quantum mechanics. The concept of shells, subshells, and
orbitals is introduced by the solution of the wave equation. The |ψ|2 at that site, where ψ denotes the wave function
of that electron, determines the likelihood of finding an electron at that
location within an atom.
Schrödinger's wave equation cannot be precisely solved
for a multi-electron atom, making its application to multi-electron atoms
challenging. Approximation techniques were used to get around this problem.
The quantum mechanical model of an atom was developed as a result of using the Schrödinger wave equation to ascertain the structure of an an atom.
This model accurately predicts the behavior and properties of atoms, including electron configurations, chemical bonding, and spectral lines. It considers the probabilistic nature of electron distribution and provides a more comprehensive understanding of atomic structure compared to earlier models.
Postulates:
Electrons are not confined to specific orbits but occupy regions of space called orbitals.
An orbital is a mathematical function that describes the probability of finding an electron in a particular location around the nucleus.
Orbitals are organized into energy levels and sublevels, based on their energy and shape.
The behavior of electrons is described by wave-particle duality, where they exhibit both particle-like and wave-like properties.
An electron can only have a fixed range of energy
values since its energy is quantized.
The Schrödinger wave equation's permitted solution,
the quantized energy of an electron, results from the electron's wave-like
characteristics.
Heisenberg's Uncertainty Principle states that it is
impossible to pinpoint an electron's precise position or momentum. As a result,
it is possible to calculate the probability of finding an electron at a given
position, which is |ψ|2 at the
location where ψ denotes the electron's wave function.
The wave function (ψ) of an electron in an atom is
known as an atomic orbital. When an electron fills an atomic orbital, it is
described by a wave function. There are numerous atomic orbitals for the
electron since it can have a wide range of wave functions. Every wave function
and atomic orbital has a specific shape and energy. The orbital wave function
of an atom's electron contains all the information about that atom's electron,
and quantum mechanics allows us to extract this information from it.
The square of the orbital wave function, or |ψ|2, at a given place within an atom, determines the likelihood of finding an electron there. Probability density, which is always positive, is denoted by |ψ|2.
First two Daltons and Bohr atomic model
No comments:
Post a Comment