Atomic structure
 |
| Atomic Structure |
Here you will learn about different atomic models that completely describe the structure of the atom, these are Dalton’s atomic theory, J.J. Thomson, Ruther Ford, Bohr, and the Quantum mechanical model of the atom.
Atomic structure
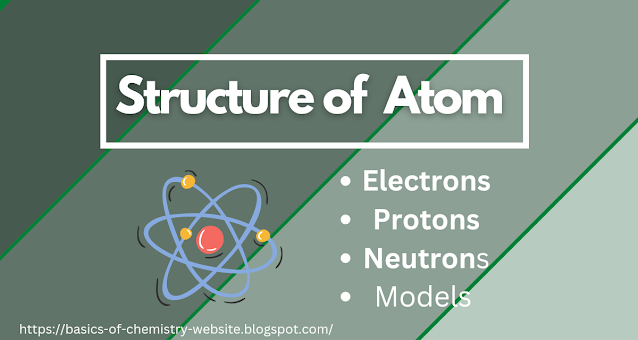
The term "atomic structure" describes the structure of an atom, which has a nucleus (center) that contains both protons (positively charged) and neutrons (neutral). The center of the nucleus is circled by negatively charged electrons.
Democritus, who initially claimed that matter is made
up of atoms, is credited with establishing the history of atomic structure and
quantum mechanics. An excellent understanding of the complete class of chemical
processes, bonds, and their physical characteristics can be gained from
studying atom structure. In the 1800s, John Dalton put forth the initial
scientific theory of atomic structure.
Atom:
Extremely small particles of matter are called atoms.
Nucleus:
The central part of an atom is called a nucleus. It
contains protons and neutrons.
Electrons:
Electrons are negatively charged atomic particles that
revolve around the nucleus of an atom. Electrons are discovered by Thomson.
Protons:
Protons are positively charged atomic particles that
are present inside the nucleus of the atom.
Neutrons:
These are particles that have no charge, these are
neutral particles present in the nucleus of an atom.
 |
| Atom |
Atomic Models
John Dalton’s theory of an atom
John Dalton, an English
physicist, and chemist proposed the atomic hypothesis in 1808 as a
scientific theory about the composition of matter. It claimed that tiny,
indivisible particles called "atoms" make up all substances.
According to Dalton's atomic
theory, atoms, which are indestructible and indivisible fundamental building
blocks, make up all substances. Unlike other elements, which had atoms of
different sizes and masses, an element's atoms all had the same size and mass.
Postulates:
Atoms are the smallest, undivided building blocks of
all substances.
A particular element's atoms are all the same mass,
size, and other characteristics. Nevertheless, the characteristics and mass of
atoms from various elements differ.
Atoms are eternal and cannot be generated or
destroyed. Atoms also cannot be separated into smaller components.
Compounds can be created by combining atoms of various
elements in particular whole-number ratios.
In chemical changes, atoms can be bonded, detached, or
modified.
 |
| John Dalton Model |
Limitations:
Dalton's atomic theory claimed that atoms were
indestructible, hence it did not take into consideration subatomic particles.
This assumption was refuted by the discovery of subatomic particles like
protons, electrons, and neutrons.
Isotopes are not taken into account. According to
Dalton's atomic theory, the masses and densities of every atom in an element
are the same. However, the atomic masses of certain element isotopes vary
(For example, hydrogen, deuterium, and tritium).
Isobars are not taken into account. According to this
idea, the masses of the atoms in two distinct elements must be different. But
two distinct elements have the same mass number. These atoms are known as
isobars (Example: 40Ar and 40Ca).
Compounds can be formed by the combination of elements
in complex, not whole-number ratios. There are some complex organic
compounds that don't have straightforward atom-to-atom ratios. Example: sucrose
or sugar (C11H22O11).
Allotropes are not taken into account by the theory. Dalton's
atomic theory is unable to account for the discrepancies between the properties
of diamond and graphite, both of which are made up entirely of carbon.
Advantages:
Dalton's atomic theory does not violate the "laws
of multiple proportions, conservation of mass, or constant proportions".
A foundation for distinguishing between elements and compounds is provided by the theory.
J.J. Thomson atomic model
William Thomson put forth the Thomson atomic model in
1900. This model provided a theoretical explanation of the description of an
atom's interior structure. Sir Joseph Thomson, who had earlier made the
discovery of the electron, backed it wholeheartedly.
J.J. Thomson found a negatively charged particle
during a cathode ray tube experiment. In 1897, this experiment was conducted. A
vacuum tube is a cathode ray tube. The electron was the name given to the
negative particle.
Thomson believed that each atom is composed of
millions of electrons and assumed that an electron is 2,000 times lighter than
a proton. He took into account atoms surrounded by a cloud that had both
positive and negative charges in his concept of the atomic structure. He and
Rutherford also performed an X-ray demonstration of the ionization of air. They
were the ones who initially showed it. The atom in Thomson's model resembles a
plum pudding.
William excluded a nucleus, protons, and neutrons from
his model.
Postulates:
A positively charged sphere with contained electrons
makes up an atom.
Because the magnitudes of the positive and negative
charges are equal, an atom as a whole is electrically neutral.
A watermelon analogy is made for the Thomson atomic
model. Where he thought:
As negatively charged particles, watermelon seeds
The watermelon's red portion is positively charged.
 |
| J. J. Thomson Model of Atom |
Limitations:
Because his atomic model did not adequately describe
how a positive charge retains the negatively charged electrons in an atom, it
was unable to explain why an atom is stable. As a result, this idea was also
unable to explain where the atom's nucleus is located.
The scattering of alpha particles by thin metal foils
was not adequately explained by Thomson's model.
There are no experimental facts to support it.
No comments:
Post a Comment