Fragmentation Pattern In Mass Spectrometry
Mass Spectrometry
In order to determine
the mass-to-charge ratio (m/z) of one or more molecules in a sample, mass
spectrometry is a valuable analytical instrument. The precise molecular weight
of the sample's constituent parts can frequently be determined using these
measures as well.
Fragmentation in mass
spectrometry is the dissociation of energetically unstable molecular ions
created when molecules pass through an instrument's ionization chamber. A
molecule's fragments result in a distinctive pattern in the mass spectrum.
A recent study has
concentrated on the fragmentation that happens during tandem mass spectrometry
investigations since this information makes molecular identification easier.
Fragmentation
Fragmentation is a
sort of chemical dissociation in which an electron is removed from a molecule,
resulting in ionization. Ionization is brought on by the removal of electrons
from sigma bonds, pi bonds, or nonbonding orbitals. This can happen by the
bond's homolytic cleavage, homolysis, heterolytic cleavage, or heterolysis. The
fragmentation process is influenced by relative bond energy and the capacity
for advantageous cyclic transition states. Stevenson's Rule outlines the
primary fragmentation mechanisms.
Simple bond cleavage
reactions and rearrangement reactions are two major groups of bond cleavage
patterns.
 |
| Fragmentation pattern |
Fragmentation reactions
Simple reactions that cleave bonds
The majority of
organic molecules proceed via simple bond cleavage reactions, which involve
direct bond cleavage. Among the many different kinds of straightforward bond
cleavage reactions are sigma bond cleavage, radical site-initiated fragmentation,
and charge site-initiated fragmentation.
Cleavage of the sigma bond
The most frequent
occurrence of sigma bond breakage is found in molecules that can create stable
cations like saturated alkanes, secondary, and tertiary carbocations. When an
alpha electron is taken away, this happens. As the C-C bond lengthens and
deteriorates, fragmentation results. At this location, fragmentation yields
both charged and neutral fragments.
 |
| Alpha Fission |
Site-initiated radical fragmentation, Homolytic cleavage
Sigma bond cleavage
can also occur on radical cations that are not ionized. Alcohols, ethers,
ketones, esters, amines, alkenes, and aromatic compounds with a carbon linked
to the ring are examples of substances where this is frequently seen. A radical
on a heteroatom or an unsaturated functional group is present in the cation.
The radical ion's significant propensity for electron pairing acts as the
catalyst for fragmentation. When the radical and an odd electron from bonds
next to the radical move to create a bond with the heteroatom or unsaturated
functional group, this is known as cleavage. This cleavage, sometimes referred
to as homolytic bond cleavage or -cleavage, occurs when the sigma bond breaks.
Heterolytic Cleavage
The inductive impact
of the charge site in radical cations is what propels fragmentation that is
triggered by the charge site. The charge-bearing atom receives electrons from
the bond next to it, which causes the charge to become neutral and shift to a
different location. This process is an illustration of heterolytic bond
cleavage and is also known as inductive cleavage.
McLafferty Rearrangement reactions
Rearrangement
reactions are fragmentation reactions that create new bonds and an intermediate
structure prior to cleavage. The McLafferty rearrangement/-hydrogen
rearrangement is one of the most researched rearrangement reactions. This
happens when radical cations, such as ketones, aldehydes, carboxylic acids,
esters, amides, olefins, and phenylalkanes, have unsaturated functional groups.
The
functional group will initially receive -hydrogen during this reaction, and the
intermediate will then undergo -bond cleavage.
 |
| McLafferty Rearrangement |
Fragmentation Rules
1. The straight chain
compound has the highest relative height of the molecular ion peak, which then
falls.
2. In a homologous
series, the relative height of the molecular ion peak often declines as
molecular weight increases. The apparent exception is fatty esters.
3.Cleavage occurs
preferentially at alkyl-substituted carbon atoms; the more substituted, the
higher the likelihood of cleavage. This results from tertiary carbocations
being more stable than secondary carbocations, which are more stable than
primary carbocations.
Tertiary > Secondary >Primary> Methyl Group
4. The molecular ion
is stabilized by double bonds, cyclic structures, and particularly aromatic (or
heteroaromatic) rings, which raises the likelihood of their appearance.
5. The
resonance-stabilized allylic carbocation is produced by double bonds, which
favor allylic cleavage. Due to the ready migration of the double bond, this
rule does not apply to simple alkenes, but it does apply to cycloalkenes.
Allylic carbon
The allylic carbon is connected to a carbon atom, which is doubly bound to another carbon atom. The allylic carbon atom is represented by the asterisk in the generic formula for allyl, which is R-CH2-CH=CH2. In contrast to the vinyl group, the allylic carbon atom is sp3 hybridised since it formed a single covalent bond with CH=CH2.
6.Alkyl side chains typically disappear from saturation rings at the bond. This is essentially an exception to branching (rule 3). The ring fragment usually retains its positive charge. A retro Diels-Alder reaction can occur in an unsaturated ring.
7. Cleavage at the
bond to the ring in alkyl-substituted aromatic compounds is very likely to
result in the resonance-stabilized benzyl ion or, more likely, the tropylium
ion. See diagram below.
8. The charge is
frequently left on the fragment containing the heteroatom, whose nonbonding
electrons offer resonance stabilization, when the C-C bonds close to it break. See below in picture.
9. Cleavage is
frequently accompanied by rearrangement and the removal of tiny, stable,
neutral molecules such alcohols, mercaptans, olefins, water, ammonia, hydrogen
cyanide, hydrogen sulphide, or carbon monoxide.
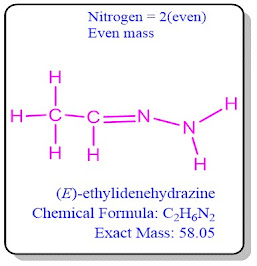
Nitrogen Rule
Any molecule (with all paired electrons)
containing an odd number of nitrogen atoms will have an odd nominal mass,
according to the nitrogen rule. The integer mass of an atom, ion, or molecule
made up exclusively of the most stable isotope is known as the nominal mass
(s).
This rule is used when molecules have only
carbon, Nitrogen, Hydrogen, Oxygen, and Halogen atoms.
Here
below are examples that are helpful for understanding the Nitrogen rule, you
will be able to find out the molecular formula of the unknown compound by using
this rule.
Compounds containing an even or odd number of nitrogen atoms their
molecular weight will also be even or odd respectively. Even or no nitrogen
atom in molecules means its molecular weight will be even.






Good Explanation
ReplyDelete